COVID-19 Update May 6, 2020
- icshealthsciencejournal

- May 6, 2020
- 5 min read
Updated: May 27, 2020
This article contains:
Promising Advancements in Technology During the Novel Coronavirus Pandemic
Understanding vaccine trials — Why it might take a long time yet for vaccinations for COVID-19 to develop
Promising Advancements in Technology During the Novel Coronavirus Pandemic
Written By: Paphapin Pairojtanachai
Across the globe, as we all fight against the novel coronavirus, scientists within the bioelectronic engineering department continue to discover new technology that contributes to a promising pathway which leads to the end of the pandemic. One of them is a dual action localized surface plasmon resonance (LSPR) biosensor that joins plasmonic sensing transduction with the photothermal effect. Swiss and Chinese researchers report that these biosensors are capable of accurately detecting airborne nucleic acids of the SARS-CoV-2 in real time. They also serve as a perfect alternative for clinical diagnosis of COVID-19 and continuous monitoring.
Dr. Wang of the Swiss Federal Laboratories for Materials Science and Technology, Dubendorf, and his team designed the biosensors with artificial DNA receptors that bind to specific sequences of the SARS-CoV-2 RNA. After testing the LSPR system, Dr. Wang certified that it can precisely identify the difference between similar RNA sequences from SARS-CoV-2 and SARS-CoV, and the results are produced within minutes. This is contrasting to the current reverse transcription polymerase chain reaction (RT-PCR), which requires many people to work with, as well as a long time before the results could be presented. Therefore, RT-PCR is not ideal for testing every single COVID-19 suspected case. Although greater improvements are needed before the LSPR biosensors can be used in public environments, the scientists confirm that they “can provide a reliable and easy-to-implement diagnosis platform to improve the diagnostic accuracy in clinical tests and relieve the pressure on PCR-based tests.
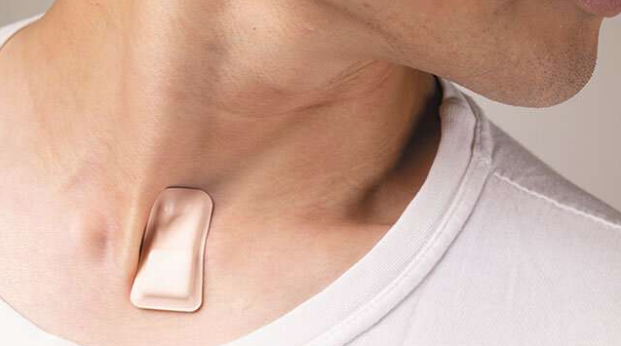
Another invention is the first wearable device that tracks COVID-19 symptoms, and it is considered to be a biotechnology breakthrough developed by researchers from Northwestern University and Shirley Ryan AbilityLab in Chicago. These medical researchers are also writing data algorithms particularly for detecting early signs of COVID-19 symptoms in suspected patients as well as for monitoring infected patients during their illness. Through the use of artificial intelligence, the device was designed to detect even the smallest change in a person’s respiratory condition, constantly producing data and cues that could save lives. Two weeks ago, 25 COVID-19 patients began using it both at home and at the clinic, collecting over 1,500 hours and one terabyte of data. This initiated a new telemedicine strategy in which patients do not have to be at hospitals for monitoring.
In addition to its ability to continuously generate data 24/7, this wearable device is also very convenient for usage due to its small size. Being soft, flexible, wireless, and thin, it fits easily into the notch at the base of the throat. Even though the device still cannot measure the level of oxygen in the blood, it can track coughing activity, chest wall movements, respiratory sounds, heart rate, and body temperature from this excellent position, and can then transfer the information to a protected cloud system which creates graphs and summaries readily monitored by healthcare workers.
Dr. Arun Jayaraman, a research scientist at Shirley Ryan AbilityLab who is leading the algorithm development, informed, “We anticipate that the advanced algorithms we are developing will extract COVID-like signs and symptoms from the raw data insights and symptoms even before individuals may perceive them.” This allows medical workers to treat patients as early as possible, thus reducing the risk of transmission and increasing the chances of fast recovery. The newly designed device definitely supplies insights at a much greater depth than any traditional machine can.
Understanding vaccine trials — Why it might take a long time yet for vaccinations for COVID-19 to develop
Written By: Kandharika Bamrungketudom
Although some politicians may be spurring hopes that the COVID-19 vaccines can be developed by the end of the year, the reality of the scientific community in the process of vaccine development may not allow it to be so, and we, as the consumer of the media, should be cautious in raising hopes too soon.
The process of vaccine development contains several stages.
The cycle starts off with the exploratory stage, where research is done to find specific antigens (whether synthetic or natural) that may be useful in disease prevention. This can include the weakened strains of viruses.
The next stage in vaccine development is the pre-clinical stage, where the vaccine candidates are tested on tissue-culture, cell-culture, or animal subjects in order to determine their effectiveness. At this stage of vaccine development, many candidates are discarded as they either prove to be ineffective or prove to be harmful.
Following the pre-clinical stage is the clinical stage, which contains three phases. In each phase, human subjects are given the candidate vaccines to test for the vaccines’ effectiveness and safety (with Phase I administering the vaccines only to less than 100 people, Phase II around 100 people and Phase III to thousands of people).
The vaccine then goes through the regulatory review and approval stage if it is to pass all three phases of the clinical trials. Following the approval, the vaccines are manufactured by drug manufacturers and distributed. However, they are still tracked for quality control, which monitors the effectiveness and safety of a vaccine that has been approved.
Looking at the COVID-19 vaccines, there is yet a long way for them to go, since out of the eight vaccines that are going through clinical trials as of May 2020, only three of them are in Phase II of the clinical trial. The reason for this is because many ethical procedures need to be considered when developing vaccines that will be used by millions of people worldwide.
Simon Kolstoe, the University Ethics Advisor at the University of Portsmouth, warns against the fake positive successful signs of vaccine researches. That is, under the pressure to produce vaccines for the novel coronavirus, the trials are instead rushed and questionable practices in the development of vaccines are used instead of following the “rigorous and reliable” procedures of good research. Kolstoe then added that it is not due to the failure of research that such vaccines cannot be produced quickly enough, but that researches have to be done with integrity, which will eventually lead to a cure over time, rather than an immediate but unsuccessful cure.
Another concern with vaccines that comes up is with the “second wave” phenomenon. When injecting a vaccine into a person, that person is exposed to the antigens of the virus that the vaccine contains. In cases such as in the Spanish Flu (1918) and dengue fever (both caused by viral infections), it has been noted that people tend to develop more severe symptoms when exposed to the antigens a second time. Due to this reason, many clinicians fear that the COVID-19 vaccines may do more harm than good, since the human body may “go into a destructive overdrive when exposed to the real virus,” after having been exposed to the antigen in the vaccine already.
Thus, the most effective treatment for COVID-19 still remains in question.








Comments